BMS-790052 (Daclatasvir)
Catalog No. A10159
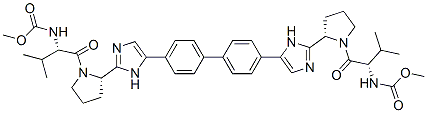
BMS-790052 (Daclatasvir)是一种高度选择性的HCV NS5A抑制剂,EC50为9-50 pM,用于治疗丙型肝炎病毒(HCV)感染。
- TS Wahyuni, .et al. Enhancement of Anti-Hepatitis C Virus Activity by the Combination of Chalepin From Ruta Angustifolia and Current Antiviral Drugs, Southeast Asian J Trop Med public health, 2020, 51(1)
- Miyayama Y, .et al. Comparative study on the replication of HCV1b genome between wild type and cell culture adaptive mutant in regard to sensitivities against anti-HCV drugs, Microbiol Immunol, 2019, Dec 19 PMID: 31854467
- Sato K, .et al. Elevated serum uric acid level was a notable adverse event during combination therapy with sofosbuvir and ribavirin, Hepatol Res, 2018, Feb;48(3):E347-E353 PMID: 28834004
| Catalog Num | A10159 |
|---|---|
| M. Wt | 738.9 |
| Formula | C40H50N8O6 |
| Purity | >98% |
| Storage | at -20°C 3 years Powder |
| CAS No. | 1214735-16-6, 1009119-64-5 |
| Synonyms | Daclatasvir |
| SMILES | CC(C)[C@@H](C(=O)N1CCC[C@H]1C2=NC=C(N2)C3=CC=C(C=C3)C4=CC=C(C=C4)C5=CN=C(N5)[C@@H]6CCCN6C(=O)[C@H](C(C)C)NC(=O)OC)NC(=O)OC |
BMS-790052 (Daclatasvir)是一种高度选择性的HCV NS5A抑制剂,EC50为9-50 pM,用于治疗丙型肝炎病毒(HCV)感染。
| In vitro | DMSO | 129 mg/mL (174.59 mM) | |
| Water | Insoluble | ||
| Ethanol | 129 mg/mL (174.59 mM) | ||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Adooq tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. |
|||
| Concentration / Solvent Volume / Mass | 1 mg | 5 mg | 10 mg |
|---|---|---|---|
| 0.1 mM | 13.53 mL | 67.67 mL | 135.34 mL |
| 0.5 mM | 2.71 mL | 13.53 mL | 27.07 mL |
| 1 mM | 1.35 mL | 6.77 mL | 13.53 mL |
| 5 mM | 0.27 mL | 1.35 mL | 2.71 mL |
*The above data is based on the productmolecular weight 738.9 . Batch specific molecular weights may vary from batch to batch due to solvent of hydration, which will affect the solvent volumes required to prepare stock solutions.



 United States
United States